For many people, to have chronic pain or treatment-resistant depression is to feel trapped. Those struggling with these conditions often report feeling “hopeless” or“despairing” when multiple therapies and interventions have not led to relief. Such people, however, describe their experience in more hopeful terms after participating in a recent study of a new biomedical technology, saying:
“This is the first time in three years I’ve felt like myself. It feels like my brain has been woken up,” or “that trauma is no longer with me. It is finally gone,” and “I was walking around the grocery store and just felt so clear. I was wondering, is this what normal people feel like?”
The contrast is clear.
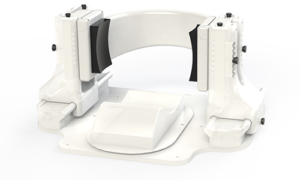
The device at the center of these human clinical trials, called Diadem, promises to change how we treat certain neurological disorders. Developed by researchers at the University of Utah and looking something like a pair of oversized headphones, it stimulates regions of the deep brain underlying chronic pain and depression — all with the power and precision of focused ultrasound.
Now, Diadem’s creators are actively seeking volunteers to participate in its final phase of clinical trials in order to obtain regulatory approval and provide these treatments to patients at a large scale.
The Cover Story
Featured on the February cover of the journal IEEE Transactions on Biomedical Engineering, their study, “Device for multifocal delivery of ultrasound into deep brain regions in humans,” represents a collaboration between the John and Marcia Price College of Engineering’s Biomedical Engineering Department and the Huntsman Mental Health Institute’s Department of Psychiatry.

In a powerful proof-of-concept study, the experiment described in the paper demonstrates focused ultrasound’s ability to stimulate deep areas of the brain and effectively reduce symptoms in patients with treatment-resistant depression. This success has already been followed up by the team’s subsequent studies using the device that further reinforces and expands this technology’s potential.
“We’ve been blown away by the positive results so far,” says the paper’s lead author Tom Riis. “After just a single 40-minute stimulation session, patients are showing immediate, clinically substantial improvements in symptoms.”
Riis, a postdoctoral researcher in the Department of Biomedical Engineering (BME), conducted this pioneering work in the lab of BME Professor Jan Kubanek, the paper’s senior author. They collaborated with Brian Mickey, professor of psychiatry at the Huntsman Mental Health Institute, PhD student Daniel Feldman, and laboratory technician Adam Losser.
Now moving to Phase 3 clinical trials, the team seeks new volunteers who struggle with treatment-resistant depression or treatment-resistant chronic pain.
 “The potential for this technique to change, even save, people’s lives is becoming increasingly clear,” Kubanek says. “We encourage those who are dealing with these conditions to join our upcoming study. It’s very important to us to get this potential treatment to those who need it as soon as possible.”
“The potential for this technique to change, even save, people’s lives is becoming increasingly clear,” Kubanek says. “We encourage those who are dealing with these conditions to join our upcoming study. It’s very important to us to get this potential treatment to those who need it as soon as possible.”
Riis adds: “And while we can’t promise remission or improvement in symptoms, we can guarantee every participant’s involvement, at the very least, will go towards pushing the science and technology forward.”
Big Results
In both the initial paper and two subsequent trials awaiting publication, one focusing on chronic pain, the other on treatment-resistant depression, the majority of patients saw improvement.
Of the 20 subjects who completed the chronic pain trial, 75% saw clinically meaningful reduction (more than 33%) in pain immediately following treatment. Of the 19 patients who completed the depression trial, 58% met criteria for remission within one week following just one stimulation session. One patient, the subject of a separate case report, remained in remission for at least 44 days.

In the chronic pain trial there were no adverse events or worsening of pain. In the depression trial, 14 patients experienced a durable improvement while 2 patients experienced a temporary worsening of depression.
“Overall, the treatments were well tolerated,” says Riis, “Regarding safety, we saw no evidence of safety issues or long-term side effects.”
While these results are promising, the researchers stress they are preliminary.
“We’ve still got a lot to learn,” Kubanek says, “more research is needed to back up the initial data and establish things like how long the improvements last.”
In other words, for the Diadem team, there’s still a long road ahead; Phase 3 clinical trials are rigorous, lasting up to two years, and only a select portion of them result in a drug or treatment being approved by the FDA. But that’s not to say the project hasn’t already made impressive strides.
And that kind of progress requires a deeper understanding of the underlying technology and physiology. To reach this point, the researchers had to confront a number of complex challenges, both on the inside and outside— and including— the human head.
Circuits, Interrupted
“The neural circuits underlying chronic pain and depression involve deep brain regions, which have been characterized by decades of careful imaging and interventional studies,” Riis says.
Targeting these sites is now possible thanks to decades of major advances in decoding and mapping the brain’s intricate tangle of 86 billion neurons; we now know where the neural circuits underlying chronic pain and depression are usually located.
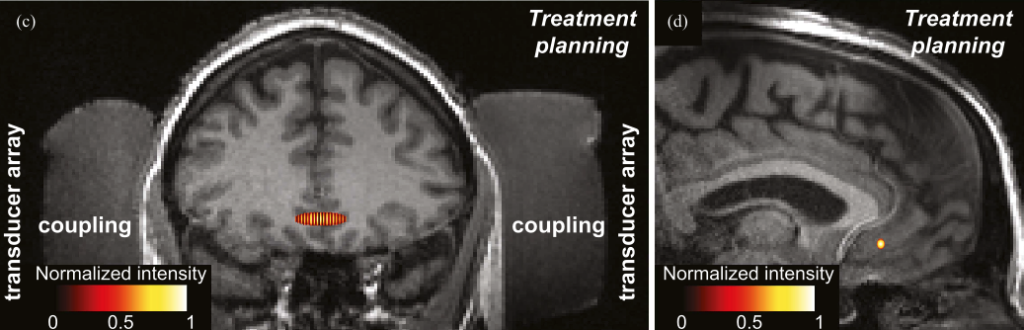
Yet even with these spots deep inside the brain identified, there’s still the question of how to access and treat them. Inconveniently for these purposes, the body protects its most powerful organ with a quarter inch layer of bone.
“You can cut open the skull and then physically manipulate the delicate brain matter inside, as with brain surgery or deep brain stimulation with electrodes, but this comes with significant risks and financial cost,” Riis says. “Traditional pharmacological approaches, on the other hand, offer a less invasive option, but at the cost of precision—acting on the brain, and body, as a whole.”
Neuromodulation
Enter: Neuromodulation, a revolutionary set of techniques that the multifocal ultrasound treatment builds upon. These therapeutic interventions involve, essentially, directing electrical, magnetic, or acoustic energy through the skull, which then targets and stimulates specific regions of the brain. Their noninvasive nature has been revolutionary in the treatment of neurological and psychiatric disorders, starting with electroconvulsive therapy (ECT) in the early twentieth century.
ECT utilizes electrical stimulation to induce seizures in the brain, effectively resetting brain waves and neural activity— and while it’s gotten a bad rap in popular culture, it was the first truly effective treatment for schizophrenia and severe mood disorders.
The late 90’s saw the advent of Transcranial magnetic stimulation (TMS), which uses magnetic pulses to alter neural activity. This has proved effective in treating major depression in some patients.
However both ECT and TMS have drawbacks, stemming from their spatial impreciseness, such as unwanted side effects or lengthy treatment cycles. Additionally, while these tools have shown to effectively treat depression, they haven’t been used for treating chronic pain.
Ultrasonic Benefits
“High-frequency sound waves, on the other hand, are non-invasive, focused, and can directly modulate the deep brain regions involved in disorders of brain function, including chronic pain,” Riis says.
“Ultrasound-based approaches can reach millimeter level precision,” he continues. “The same way you can focus light through a magnifying glass, you can focus sound waves into a small, intense volume. So we can stimulate a region about the volume of a peanut anywhere we want within the brain.”
But the skull still presents a formidable challenge. Its variable thickness and irregular shape scatters and distorts sound waves, greatly hindering attempts to precisely target small regions of the deep brain.
The team’s technical breakthrough was in enabling the Diadem device to take into account the many “acoustical distortions” caused by the skull. Understanding how those distortions change the shape and trajectory of sound waves allows Diadem to correctly target those “peanut-sized” areas, even if they are deep inside the brain, without the need to move the device or the subject.
This is achieved with a total of 252 separate transducers — effectively, individual wave emitters— that are divided between Diadem’s two transducer arrays, one placed on each side of the skull where the bone is thinnest. By overcoming the acoustic complexities of the human skull, the Diadem device can activate deep brain neurons and glial cells in a controlled, deterministic way.
Critically, the researchers expect to show that Diadem’s ability to precisely modulate activity in these deep brain structures can make it effective in reducing symptoms in some patients after a single treatment session.
Looking Forward
For all the complex science behind the device, patients’ clinical experience with Diadem is relatively straightforward.
In a preliminary visit the apparatus is individually fitted to the patient’s head and a single MRI scan is taken to get an image of the brain. In the next session outside the scanner, the researchers, using the previous MRI scan as a guide, set the device to target the correct spot that underlies a patient’s condition. Ultrasonic stimulation is then delivered to that area for about 40 minutes, with the aim of modulating the neural activity that was causing the patient’s symptoms. This can be repeated for multiple sessions for more effect or to different areas of the brain until the patient sees improvement in symptoms.
“While it should be kept in mind that not every participant saw drastic improvement, in the ones that did the change could be quite remarkable,” says Riis.
“For several you could just see it in their eyes— coming out of the session, their mood and behavior were a total 180 from when they had walked in. They were noticeably at ease, less burdened, more present.”
Kubanek adds: “These are patients who have been dealing with pain most of their life, or have tried dozens of antidepressants with no relief, or have been hospitalized for suicidal ideation multiple times. So seeing the results of this drug-free approach has been very rewarding to us as researchers.”
“Honestly, this is probably the most meaningful work I’ve ever done,” Riis says. “It’s not very often you get to see your work getting out of the lab and making such a clear and powerful difference in people’s lives.”
With the large-scale Phase 3 trials on the horizon — the final step before FDA approval to use Diadem as a treatment for the general public — that impact should only grow.
“We again encourage those dealing with these conditions to not hesitate to reach out; we’re going to need many participants for the final Phase 3,” says Kubanek. “This is a chance to help tackle these recalcitrant disorders.”
To participate in the Diadem Device’s next phase of clinical trials, please Email
DIADEMCLINICALTRIALS@GMAIL.COM