
Portable electronic devices aren’t the only things that benefit from batteries. Buildings, including warehouses, shopping centers, hospitals and police stations, use large stationary batteries in case of power outages or to store electricity generated from rooftop solar panels. And as we move toward cleaner sources of energy such as solar and wind, these kinds of large-capacity batteries will be even more important.
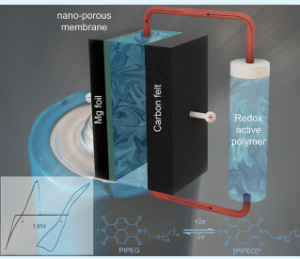
University of Utah chemical engineering assistant professor Tao Gao has demonstrated that a new kind of high-voltage flow battery can be made with magnesium and a polymer that can store more energy at a lower price than standard batteries used today.
His discovery is described in the new paper, “Nonaqueous Mg Flow Battery with a Polymer Catholyte,” in the latest issue of ACS Applied Energy Materials. Co-authors on the paper include U chemical engineering graduate student Yunan Qin and undergraduate student Dillon Fehlau, George Mason University chemistry and biochemistry assistant professor Chao Luo and George Mason chemistry and biochemistry graduate student Kathryn Holguin.

“This is the first work on a magnesium-polymer flow battery in the field,” Gao said. “It demonstrates the concept works — that we can indeed make a high-voltage flow battery by pairing the magnesium metal with a polymer solution.”
For energy storage, buildings use either lithium-ion batteries, lead-acid batteries or zinc- bromine batteries, but those solutions are either more expensive (lithium-ion) or the chemicals are toxic, he said. But Gao’s magnesium-polymer solution could potentially store as much as two to three times the amount of energy as lead-acid and zinc-bromine at a lower cost because of the abundance of magnesium and could become more sustainable.
In a rechargeable flow battery, electrolyte flows through one or more electrochemical cells to create electricity. Gao and his team have created a novel polymer material that is both soluble and can react reversibly with magnesium.
 “There are still some challenges that need to be addressed before it becomes commercially relevant,” he added, however. “For example, we still need to increase the cycle life of the battery, we still need to make the polymer more soluble, etc.”
“There are still some challenges that need to be addressed before it becomes commercially relevant,” he added, however. “For example, we still need to increase the cycle life of the battery, we still need to make the polymer more soluble, etc.”
But once fully developed, this new battery technology could benefit buildings that use emergency backup battery power by providing more energy during blackouts and at a lower cost. Gao said this concept could not be used in portable electronic devices that use lithium-ion batteries because those kinds of devices require a lot of energy packed into a much smaller volume while stationary energy storage requires both low cost as well as long service life.
Click here to read a copy of the paper.